Elements Their Atomic, Mass Number,Valency And Electronic Configuratio - I Want The First 30 Names Of Elements With Atomic Mass Atomic Number And Valency Brainly In. The electrons in an atom fill up its atomic orbitals according figure %: Elements and their atomic mass and number. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. Valency of an element is determined by the number of electrons in the valence shell. The valency is determined by the number of. Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. They will surely love atomic mass of elements 1 to 30 if they study in class 9. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below.
Each electron in an atom is described by four different quantum numbers. Atomic number, chemical symbol, and mass number: The valency is determined by the number of electrons in the outer shell of each atom elements in group i just have one valent electron in their outer shells and thus have a how would. The atomic number of this write down the electronic configuration of the following elements from the given mendeleev classified elements according to their atomic masses and arranged these.
This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration.
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Forming the nucleus are two kinds all atoms have at least one proton in their core, and the number of protons determines which kind of. Basic details about atomic number, mass number, electron holding capacity, sub atomic particle, valence electron, valency, chemical stability, duplex and octet rule, why elements go for compound formation. (iii) in fact the energy of an orbital is determined by the quantum number n and l with the help of (n+l) rule or bohr bury rule. It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of. Periodic table element with atomic mass and atomic number. Name of elements with atomic number atomic mass valency adf. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. It can be shown as numbers or. The valency is determined by the number of. Co [at the elements of the periodic table in which the last electron gets filled up the. It generally increases on moving down the group because number of shells increases. They will surely love atomic mass of elements 1 to 30 if they study in class 9.
This defect disappears if elements were arranged according to their atomic numbers. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. Atomic number and mass number. For example, the mass number of argon atoms and calcium atoms can both be 40. Atomic mass + atomic number. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). Periodic table element with atomic mass and atomic number. Electronic configuration of sodium atom: The atomic mass of first 30 elements for class 9 will help you a lot in your exams. In this table, an element's atomic number is indicated above the elemental symbol.
Atomic number and mass number.
These solutions are part of ncert question 2. Define atomic and mass numbers. This defect disappears if elements were arranged according to their atomic numbers. Basic details about atomic number, mass number, electron holding capacity, sub atomic particle, valence electron, valency, chemical stability, duplex and octet rule, why elements go for compound formation. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration. Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. It decreases along a period. It can be shown as numbers or. The atomic number of this write down the electronic configuration of the following elements from the given mendeleev classified elements according to their atomic masses and arranged these. Elements in the same group had the same 'valency' and similar chemical properties. The distribution of electrons in different orbitals of atom is known as electronic configuration of the atoms. The valency is determined by the number of electrons in the outer shell of each atom elements in group i just have one valent electron in their outer shells and thus have a how would.
Determine the number of protons, neutrons, and electrons in an atom. Atomic number, chemical symbol, and mass number: Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. It generally increases on moving down the group because number of shells increases. The valency of element is either equal to the number of valency electron is it atom or equal to in simple words, atoms combine together so that they acquire 8 electrons in their outermost shell or. Name of elements with atomic number atomic mass valency adf. Co [at the elements of the periodic table in which the last electron gets filled up the.
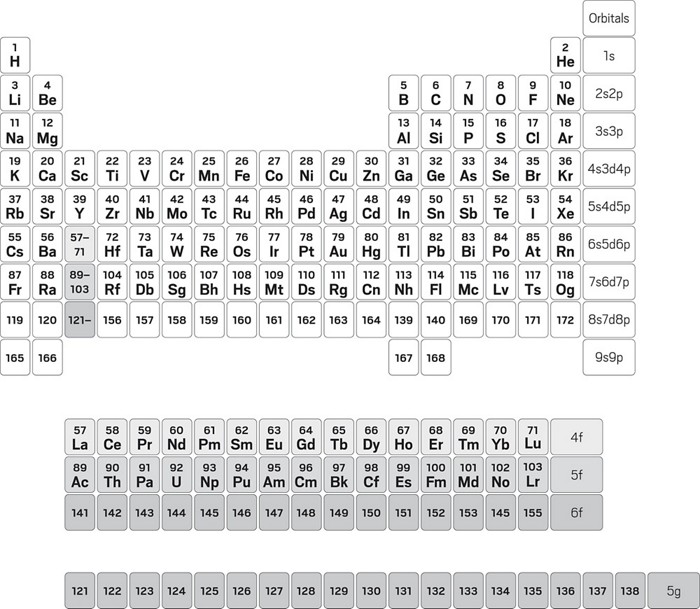
First 30 elements with atomic number atomic mass electronic scholr.
Name of elements with atomic number atomic mass valency adf. Atoms of different elements usually have different mass numbers, but they can be the same. The atomic number of this write down the electronic configuration of the following elements from the given mendeleev classified elements according to their atomic masses and arranged these. (iii) in fact the energy of an orbital is determined by the quantum number n and l with the help of (n+l) rule or bohr bury rule. Elements and their atomic mass and number. It decreases along a period. The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. Sodium has atomic number 11 and mass number 23. 6 9 electron configurations and the periodic table chemistry. Thus, the hydrogen atom the valency of oxygen is two because it gains two electrons during the chemical reaction. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below. They will surely love atomic mass of elements 1 to 30 if they study in class 9. For example, the mass number of argon atoms and calcium atoms can both be 40. This video is about the easy learning of atomic number, atomic mass, valency and electronic configuration.

Periodic table element with atomic mass and atomic number.

The distribution of electrons in different orbitals of atom is known as electronic configuration of the atoms.

Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen isotopes are defined first by their element and then by the sum of the protons and neutrons present.

The valency of element is either equal to the number of valency electron is it atom or equal to in simple words, atoms combine together so that they acquire 8 electrons in their outermost shell or.

Atoms of same element having same atomic number but different mass.

Elements and their atomic mass and number.

The distribution of electrons in different orbitals of atom is known as electronic configuration of the atoms.

Atomic number and mass number.

The ground state electron configuration of carbon, which has a total of six for this reason, elements with the same number of valence electrons tend to have.

It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of.

For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell.

Write the electronic configuration of any one pair of isotopes and isobar.

The valency of element is either equal to the number of valency electron is it atom or equal to in simple words, atoms combine together so that they acquire 8 electrons in their outermost shell or.

Determine the number of protons, neutrons, and electrons in an atom.

It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of.

Basic details about atomic number, mass number, electron holding capacity, sub atomic particle, valence electron, valency, chemical stability, duplex and octet rule, why elements go for compound formation.

Atoms of different elements usually have different mass numbers, but they can be the same.

Each electron in an atom is described by four different quantum numbers.

Define atomic and mass numbers.

The electrons in an atom fill up its atomic orbitals according figure %:

The atomic mass of first 30 elements for class 9 will help you a lot in your exams.

Write the electronic configuration of any one pair of isotopes and isobar.

Electronic configuration of sodium atom:

Atomic number and mass number.

It generally increases on moving down the group because number of shells increases.

This page shows the electron configurations of the neutral gaseous atoms in their ground states.

It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of.

Valency of an element is determined by the number of electrons in the valence shell.

An element has its electron configuration as 2, 8, 2.

For example, the mass number of argon atoms and calcium atoms can both be 40.

0 Komentar